![]()
![]()
![]()
![]()
Honey bees build honeycombs of wax that exhibit a hexagonal tiling. Why hexagons? As this Ted Ed video explains, the hexagonal structure utilizes space most efficiently and uses the minimal amount of wax to create a given number of columns per volume.
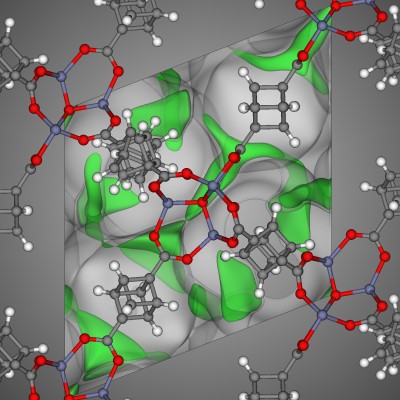
In materials science, the crystal structure of a well-studied nanoporous material, MOF-74 [1], resembles a honeycomb; the structure consists of 1-dimensional channels with a hexagonal cross-section.
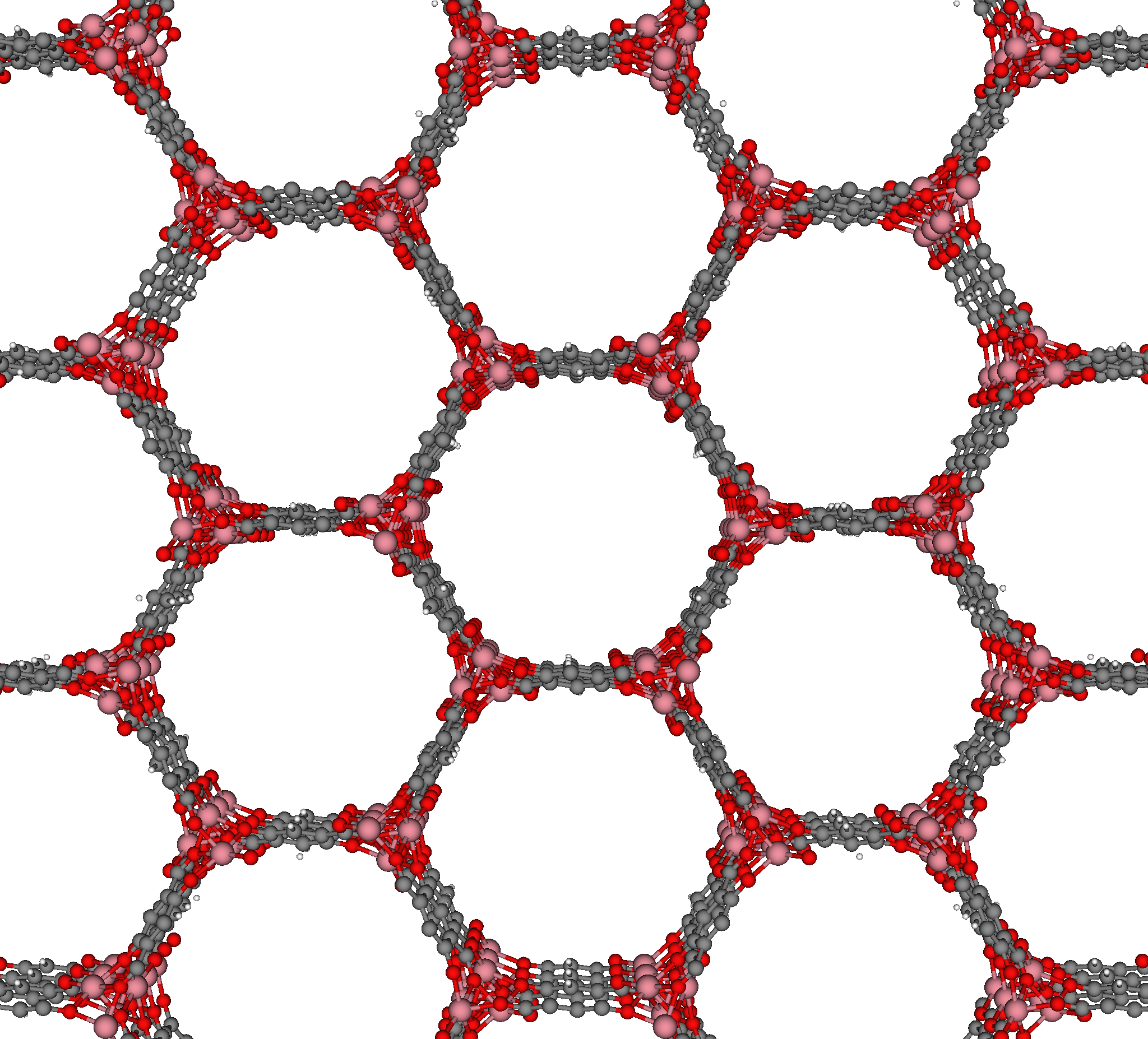
The crystal structure of MOF-74 constructs a surface that, like the honeycomb, most efficiently utilizes a volume to create a given number of channels, while minimizing the amount of material required.
Instead of storing honey, MOF-74 can be used to store or separate gas molecules. For example, MOF-74 is a promising candidate to selectively capture the greenhouse gas carbon dioxide from the exhaust of coal-fired power plants [2]. The carbon dioxide can then be compressed and pumped underground to be stored in geological formations, where it cannot instigate climate change.

[1] Rosi, N. L., Kim, J., Eddaoudi, M., Chen, B., O’Keeffe, M., & Yaghi, O. M. (2005). Rod packings and metal-organic frameworks constructed from rod-shaped secondary building units. Journal of the American Chemical Society, 127(5), 1504-1518.
[2] Britt, D., Furukawa, H., Wang, B., Glover, T. G., & Yaghi, O. M. (2009). Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites. Proceedings of the National Academy of Sciences, 106(49), 20637-20640.
comments powered by Disqus